Formulation R&D Platform
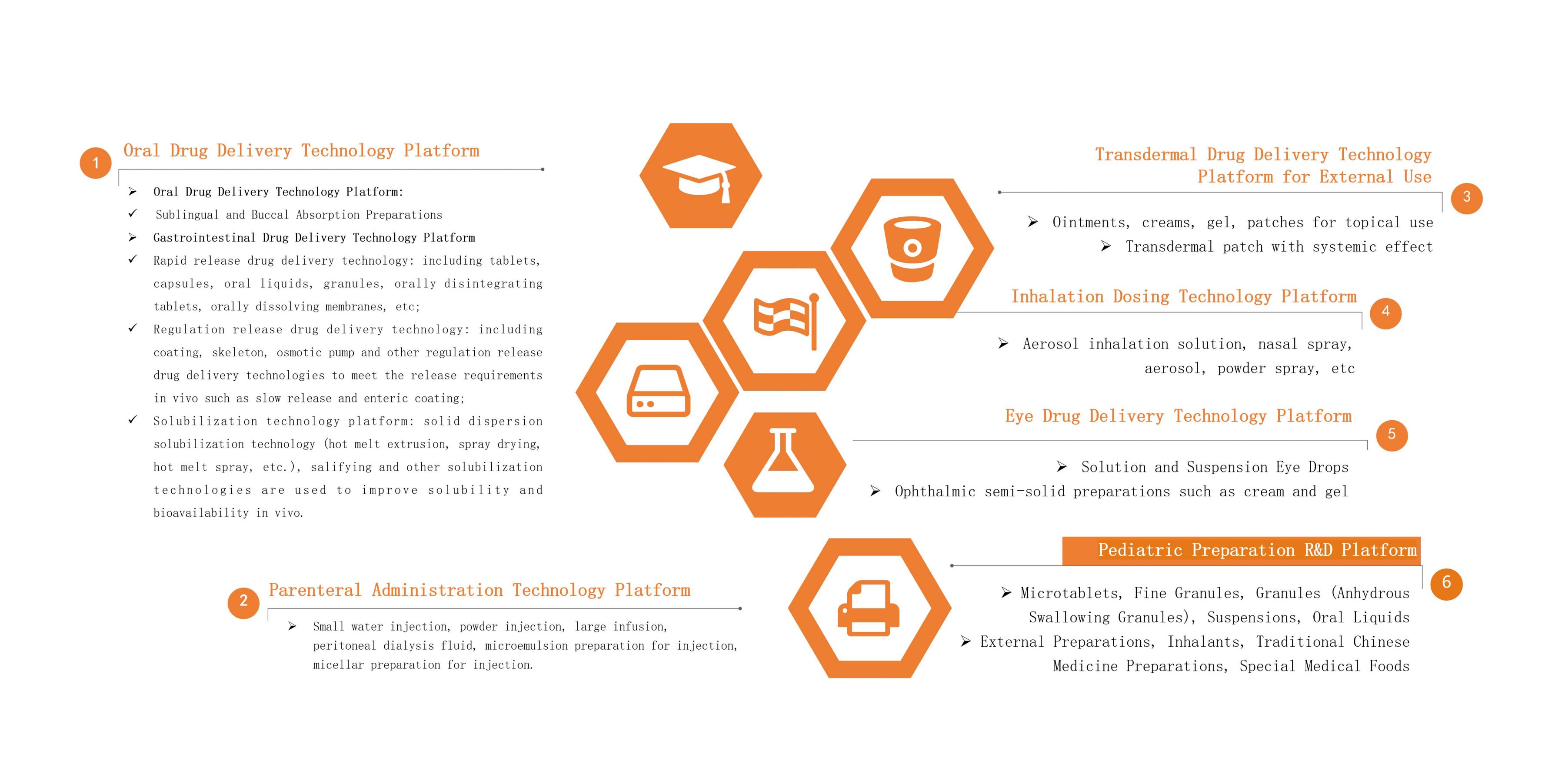



Oral Drug Delivery Technology Platform
The Oral Drug Delivery Technology Platform of Leadingpharm has a research and development team of nearly 200 people. In addition to ordinary oral solid preparations and oral liquid preparations, it can carry out research on sublingual/mucosal absorption preparations, high-end controlled-release preparations, gastrointestinal targeted preparations, and provide solubilization technical services.
♦Gastrointestinal-Targeted Drug Delivery
♦Solubilization Technology
♦ Modified-Release Preparations



Parenteral Administration Technology Platform
The Parenteral Drug Delivery Technology Platform has a research and development team of nearly 200 people. In addition to conventional water injection, powder injection, infusion, oral liquid, peritoneal dialysis fluid and other liquid preparations, it can also carry out research projects such as microemulsion preparations for injection, micellar preparations for injection.
♦ Eye Drug Delivery Technology Platform
The Ophthalmic Drug Delivery Technology Platform focuses on the clinical needs of ophthalmic drug delivery, and cooperates with the industry's senior expert think tank to carry out research on ophthalmic semi-solid preparations including solution type and suspension type eye drops, creams, gel, etc.


Inhalation Dosing Delivery Technology Platform
The inhalation platform can carry out the development, industrialization technology transfer and registration services of generic drugs and improved innovative drugs for inhalation preparations, providing customers with complete solutions.
The dosage forms mainly involves inhalation aerosol (MDI), inhalation powder aerosol (DPI), inhalation liquid preparation (nebulized inhalation solution), nasal spray. At present, there are more than 20 varieties under research, many varieties have entered the industrialization stage or IND stage, can be approved transfer or in-depth cooperation, such as ipratropium bromide solution for inhalation, levosalbutamol solution for inhalation, raphenacin solution for inhalation, moxifloxacin hydrochloride solution for inhalation, etc., which can efficiently provide customers with services such as registration, approval document acquisition, and value conversion.
Equipped with international advanced equipment including liquid dispensing system (with heating and cooling function), bladder filter, high shear homogenizer, microjet nano homogenizer, ampoule melting and sealing machine, automatic autoclave, blow filling (BFS) integrated machine, etc. Equipped with good atomization characteristic detection equipment, respiratory simulator, pharmaceutical multistage impactor (NGI), laser diffraction particle size meter, etc., it has a stability research system and facilities that meet GMP conditions to ensure the integrity and reliability of R&D data.




Transdermal Drug Delivery Technology Platform for External Use
The Transdermal Drug Delivery Platform is committed to solving the technical barriers of transdermal drug delivery preparations, improving the quality and efficacy of products, and providing pharmaceutical and clinical services for transdermal drug delivery preparations at home and abroad.
At present, the R&D team has nearly 30 people, all from first-class universities at home and abroad, and has more than 10 years experience of pharmaceutical R&D. The instrument and equipment are equipped with vacuum homogenizing emulsification machine, full-automatic metal tube filling and sealing machine, transdermal diffusion meter, cone penetration tester, peeling force tester, cone plate viscometer, rotary rheometer, particle size analyzer, polarizing microscope, coating machine, emulsification machine, adhesion tester, initial adhesion tester, adhesion tester, etc., which can meet the requirements of preparation research and development. It can carry out the research on chemical external lotion, gel, paste, patch and other dosage forms. Besides, it can and provide clinical research, registration services, etc.
The platform has carried out the research and development of several chemical drugs for external use and two Class II new drugs for external use. The projects under research include Loxoprofen Sodium Patch, Amorofen Hydrochloride Liniment, Lidocaine Dicaine Cream, Lidocaine Tetracaine Cream, Pimecrolimus Cream, Criborol Ointment, Lidocaine Gel Patch, Fentanyl Transdermal Patch, Fusidic Acid Cream, Minoxidil Topical Solution, Metronidazole Gel, etc.

 Hot line:010-83057670
Hot line:010-83057670
 EN
EN
 010-83057670
010-83057670 Address:
Address: Marketing Department:
Marketing Department: Leadingpharm
Leadingpharm Pharm News
Pharm News 010-61006450
010-61006450
